The World Health Organization updated its guidelines for essential medicines Wednesday to include drugs to treat multiple sclerosis, heart conditions, cancer and more. But obesity medications were not added to the list, despite requests this year from researchers in the United States.
During its meeting in April, WHO’s review committee considered over 100 therapeutics before recommending 24 medicines for adults and 12 for children to be added to the Model Lists of Essential Medicines (EML) and Essential Medicines for Children (EMLc).
The additions bring the total number of medications on the EML and EMLc to 502 and 361, respectively.
Updated every two years, the lists are registers of medications that WHO considers to be minimum requirements for every health care system to have available. The lists are internationally recognized guides for countries’ health systems, helping them prioritize medications that are effective and affordable. Each addition, according to WHO, is considered “essential to address key public health needs.”
“Essential medicines are those that satisfy the priority health care needs of a population,” the report says. “They are intended to be available in functioning health systems at all times, in appropriate dosage forms, of assured quality and at prices individuals and health systems can afford.”
New on the 2023 list are medications to treat multiple sclerosis, or MS, a chronic, often fatal nervous system disease that affects 2.8 million people around the world, according to WHO. The new guidelines include three medications to slow its progression.
One of them, rituximab, is normally used to treat some cancers and autoimmune diseases, but the WHO guidelines recommend off-label use for MS due to “strong evidence of its efficacy and safety.”
“Given the evidence base and the increased affordability of rituximab … it has been prioritized over on-label alternatives as an essential medicine to treat relapsing-remitting and progressive MS,” said Dr. Benedikt Huttner, the EML team lead, in a statement.
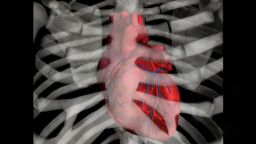
The guidelines also recommend, for the first time, “polypills” to treat heart disease and other cardiovascular issues. The term refers to a combination of medicines to treat heart issues: medication to lower blood pressure, a statin to lower cholesterol, a medication to make the heart beat with less force and sometimes aspirin.
A growing body of research shows that polypills can be an inexpensive, effective way to reduce the risk of heart problems, with studies indicating that they can cut the risk of cardiovascular problems by almost 40%. But even though heart complications like heart disease and heart attacks kill 18 million people each year, only a few companies manufacture polypills, and few people take them.
The inclusion of polypills on the WHO guidelines could change that. Some health officials believe that their place on the Essential Medicines List would encourage governments and insurance companies to recommend them, particularly in low- and middle-income countries.
“These treatments could have a very large public health impact globally, without jeopardizing the health budgets of low- and middle-income countries,” said Dr. Tedros Adhanom Ghebreyesus, WHO’s director-general, in a news briefing Wednesday.
WHO rejected the inclusion of several patented cancer medications due to concerns over their high price, but the guidelines did add two cancer treatments: a medication for Kaposi sarcoma, a cancer that causes lesions in the skin and gastrointestinal tract, and cancer treatments that improve white blood cell production and reduce some cancer medicines’ toxic effects on the bone marrow.
Other additions include ceftolozane and tazobactam, a combination antibiotic used to treat multidrug-resistant bacteria, and monoclonal antibodies against Ebola.
Notably absent from the Essential Medicines List are compounds called GLP-1 receptor agonists, commonly used in some diabetes and obesity medications like Ozempic and Wegovy.
A request to add GLP-1 receptor agonists to the list came in March from four researchers at Yale University, Brigham and Women’s Hospital, and the University of California, San Francisco. However, the WHO committee rejected the application, citing the compounds’ “uncertain long-term clinical benefit and safety in this patient population.”
Get CNN Health's weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
Amid celebrity promotion and their rising popularity for weight loss, some medications containing GLP-1 receptor agonists have been in shortage in the United States.
Shortages have also affected several essential medications on WHO’s list, including the hormone insulin for diabetes control and the antibiotic bicillin, a long-acting injectable form of penicillin.
“Rising prices and supply chain disruptions mean that all countries now face increasing problems in ensuring consistent and equitable access to many quality-assured essential medicines,” Ghebreyesus said. “WHO is committed to supporting all countries to overcome these obstacles to increase equitable access to essential medicines.”
WHO updates list of essential medicines to include heart ‘polypills,’ MS treatments but not weight-loss drugs - CNN
Read More


No comments:
Post a Comment