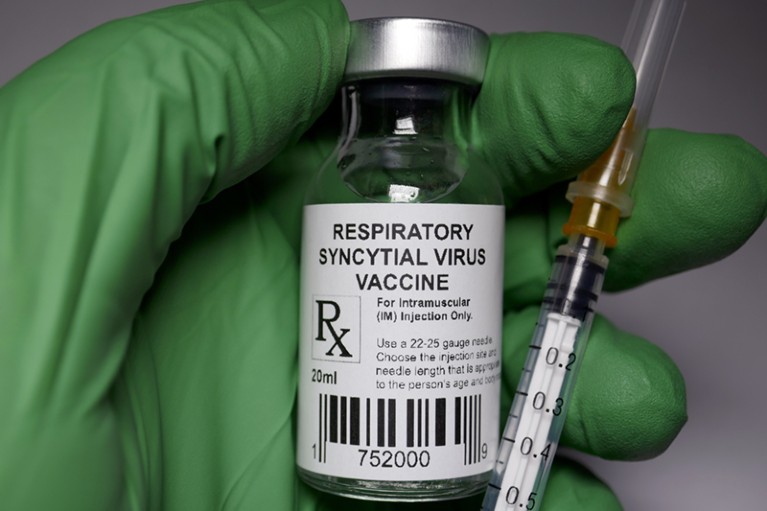
The US Food and Drug Administration (FDA) has approved GSK’s vaccine against respiratory syncytial virus (RSV) for use in people 60 years and older. This is the first RSV vaccine to gain approval anywhere in the world, and researchers are celebrating.
“It's a very big deal to have options available to prevent RSV disease,” says Barney Graham, senior adviser for global-health-equity trials at Morehouse School of Medicine in Atlanta, Georgia.
Will a new wave of RSV vaccines stop the dangerous virus?
RSV usually causes mild symptoms like those that arise during a common cold. But for older people, it can be deadly. According to the US Centers for Disease Control and Prevention (CDC), each year RSV kills approximately 6,000 to 10,000 adults in the United States who are 65 years and older, and sends 60,000 to 160,000 to hospital. People in this age group with comorbidities such as chronic obstructive pulmonary disease, asthma and congestive heart failure are especially at risk.
The FDA approved GSK’s RSV vaccine, to be sold as Arexvy, on the basis of phase III clinical-trial data submitted by the pharmaceutical company, which is based in Brentford, UK. Those data showed that its shot reduced the risk of people 60 and older developing lower respiratory tract disease from RSV by 82.6% and the risk of developing severe disease by 94.1%. The results were published earlier this year in The New England Journal of Medicine1.
The approval is “a tremendous opportunity to help address a really important public health need,” says Leonard Friedland, vice president and director of scientific affairs and public health for GSK’s US vaccines division.
A life’s work
The FDA expedited the approval of GSK’s vaccine last November, during a period when the United States was grappling with a ‘tripledemic’ of people with RSV, influenza and COVID-19 flooding hospitals.
The race to make vaccines for a dangerous respiratory virus
The technology underlying the RSV vaccine has been almost 60 years in the making. In the 1960s, a clinical trial of an RSV vaccine killed two of the participating children and sent 80% of them to hospital. Understanding what happened and finding a solution became Graham’s life’s work. “The first 20 years were spent primarily working out how to make a vaccine that could be safe,” he says.
The original vaccine contained an inactivated RSV virus. Eventually, the vaccine research community pivoted its strategy.
In 2008, Graham teamed up with Jason McClellan and Peter Kwong at the US National Institutes of Health (NIH) in Bethesda, Maryland, and others to investigate the structural biology of the RSV virus. Over time, they learnt that the virus used protein F, a molecule on its surface, to infect a person’s cells. With this knowledge, protein-based RSV vaccines could be developed that would introduce the protein into cells to elicit an immune response.
But the team eventually found that early jabs of this type were being designed around the wrong form of protein F, a ‘postfusion’ form that arises after the virus and cell have already joined. The researchers discovered a way to instead target the correct form of protein F, a version that hadn’t yet fully fused with any cell and could elicit neutralizing antibodies. They published their findings in 20132.
Their work paved the way for companies such as GSK, Pfizer and Moderna to develop the RSV vaccines in the pipeline today. “This is my lifetime's work. It’s very gratifying to see this finally happening,” Graham says. “It’s a good day for RSV.”
The race continues
After the approval of GSK’s vaccine, experts hope others will follow. “There are many patients all around the world who can benefit from vaccination,” Friedland says.
RSV wave hammers hospitals — but vaccines and treatments are coming
The pharmaceutical firm Pfizer, based in Manhattan, New York, has a protein-based RSV vaccine for people 60 and up that the FDA is expected to approve later this month. Biotechnology company Moderna in Cambridge, Massachusetts, also has an mRNA-based vaccine for preventing RSV in adults 60 and older under expedited review with the agency.
And an FDA advisory panel will convene on 18 May to mull the safety and effectiveness of Pfizer’s RSV vaccine for pregnant people. During a phase III clinical trial, pregnant people to whom the jab was administered gave birth to newborns who were then monitored for illness. The vaccine reduced the risk of infants up to 90 days old acquiring severe lower respiratory tract illness from RSV by 81.8%3. The FDA will decide whether to approve the jab by August.
Like older adults, babies are at high risk from RSV. “RSV seems like a harmless thing,” but it’s not, says Mina Suh, a scientist at the company EpidStrategies in Rockville, Maryland, that evaluates epidemiological studies. Each year, 58,000 to 80,000 children in the United States 5 years and younger are hospitalized because of RSV and 100 to 300 die, according to the CDC.
Newborn babies’ immune systems don’t respond robustly to many vaccines, so it is challenging to give them shots directly. To protect infants, Pfizer adopted the strategy to immunize pregnant people a few months before birth. Their bodies make the antibodies that are transferred to the newborn.
But before this tranche of vaccines is approved, GSK’s jab will move ahead. It is expected to be approved in Europe soon. And the next step in the US process is for the CDC's Advisory Committee on Immunization Practices (ACIP) to weigh in. It will recommend who can receive the GSK jab, how and when. ACIP’s next meeting is in June. This is an important step, Friedland says. “Vaccines don’t save lives. It’s vaccination that does.”
'A good day': FDA approves world's first RSV vaccine - Nature.com
Read More

No comments:
Post a Comment