-
Herpes simplex virus (HSV) 1 and 2 infections primarily cause oral or genital disease that is usually self-limited or resolves with antiviral therapy.
-
Hypertrophic HSV infection is an uncommon manifestation that most frequently affects anogenital structures and is typically seen in immunocompromised patients.
-
Acyclovir and its related compounds, valacyclovir and famciclovir, are the primary antiviral agents used to treat HSV infections.
-
Resistance should be suspected in patients who do not clinically respond to first-line treatment, especially in those who are immunocompromised and those with repeated exposures to these medications.
-
Antiviral options for patients with resistance include foscarnet and cidofovir.
An 80-year-old man on ruxolitinib for myelofibrosis was referred to the infectious diseases clinic with a subacute, progressive mass over his left forehead. He also had type 2 diabetes mellitus and dyslipidemia, and was taking rabeprazole, simvastatin and metformin.
Three years before presentation, he developed an erythematous, crusting rash over the outer side of his left ear. He was previously given a diagnosis of otitis externa, but the rash did not improve despite 14 empiric courses of oral antibacterial therapy. A swab from the lesion was sent for herpes simplex virus (HSV) testing by polymerase chain reaction (PCR), which was positive for HSV-2. The patient had no history of oral or genital HSV infection. The rash resolved with a 5-day course of oral valacyclovir (1 g, 3 times daily). Over the following 3 years, the patient had 8 recurrences involving the left side of his face. These were presumed to be episodes of HSV-2 reactivation and each resolved with empiric oral valacyclovir for 7–10 days.
Six months before presentation, the patient developed a small, sessile, sesame seed–shaped lesion over his left forehead. Despite 18 courses of oral valacyclovir and 3 courses of oral famciclovir (500 mg, twice daily), each for 7–14 days, the mass continued to increase in size. A biopsy was performed, and viral culture was positive for HSV-2. Histopathology showed acantholytic keratinocytic cells with viral changes, suggestive of an ulcerative lesion of viral etiology.
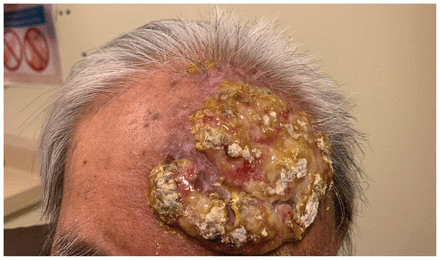
When we saw the patient in clinic, he had a fungating, verrucous mass on his left forehead measuring about 12 × 8 cm and extending superiorly to the scalp (Figure 1). The mass was raised and pink, with a well-demarcated border. It had regions of slough and crusting, but was not tender. The mass and associated edema resulted in slight left-sided ptosis. The patient had no other cutaneous lesions on the head and neck. Cranial nerve examination was normal. Laboratory investigations showed leukocytosis and anemia that were caused by his myelofibrosis (leukocyte count 17.1 [normal 4–11] × 109/L and hemoglobin 95 [normal 120–160] g/L). His creatinine was 92 (normal 42–102) μmol/L.
A large fungating and verrucous lesion on the left forehead of an 80-year-old man, caused by herpes simplex virus 2 infection; the lesion was progressive over a 6-month period.
Based on the patient’s history, including multiple previous courses of antiviral treatment, our presumptive diagnosis was hypertrophic HSV infection, with concern for resistance to acyclovir and related compounds (valacyclovir and famciclovir), as evidenced by the lack of clinical response. We obtained a swab of the mass for HSV PCR, which was positive for HSV-2. Genotyping was performed at the National Microbiology Laboratory in Winnipeg. Sequence variations in the UL23 thymidine kinase gene were identified, confirming resistance. Testing for HIV was negative.
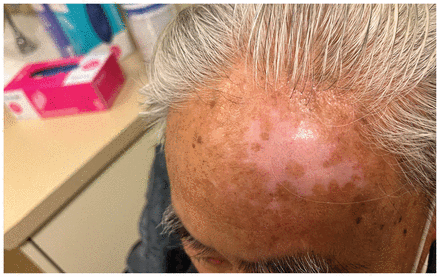
We treated the patient with intravenous foscarnet (90 mg/kg, daily) in our hospital’s infusion clinic. He received 20 doses in total, with substantial improvement (Figure 2). By the end of therapy, the lesion had flattened and regressed in diameter, with a residual irregularly shaped region of hypopigmented skin. The patient had 2 further recurrences on his left ear 3 and 11 months after his initial treatment. Each responded to foscarnet. Given the resistance to acyclovir and related compounds, no oral antiviral options were available for suppressive therapy. If the patient has additional recurrences, further management strategies will include immune modulation therapy with topical imiquimod.
Left forehead of an 80-year-old man with herpes simplex virus 2 infection after treatment with intravenous foscarnet, showing flattening and regression of the mass, with areas of postinflammatory hypopigmentation.
Discussion
Herpes simplex virus 1 and 2 belong to the Herpesviridae family of DNA viruses. Infection with HSV is common; estimated seroprevalences among adults in Ontario are 51.1% for HSV-1 and 9.1% for HSV-2.1 The 2 primary clinical manifestations are oral and genital infection. Classically, HSV-1 is associated with oral infection and HSV-2 with genital infection, but the reverse trend is occurring with greater frequency.2 As with all Herpesviridae, HSV-1 and HSV-2 have the capacity for latency and can reactivate intermittently after primary infection. Primary oral infection can be severe, characterized by painful gingivostomatitis and pharyngitis, with exudative, ulcerative lesions of the oropharynx. Recurrences tend to be mild and are characterized by painful vesicular lesions, classically located at the vermillion border. Similarly, primary genital infection is typically more severe, with bilateral, painful, ulcerative lesions, regional lymphadenopathy and systemic symptoms (such as fever, headache and malaise). Recurrent genital infection is usually less severe, with painful, unilateral, vesicular and ulcerative lesions. Other cutaneous manifestations include herpetic whitlow and herpes gladiatorum, the latter of which occurs in the setting of contact sports.3 Herpes simplex virus 1 and 2 can also cause infection at other sites, such as the anus and perianal skin, particularly among men who have sex with men (MSM).
Mucocutaneous HSV infections are typically diagnosed by HSV PCR of swabs obtained from herpetic lesions. Acyclovir and related compounds are first-line therapies (Table 1).4 Treatment is associated with reduced symptom duration and decreased risk of transmission, and should be started as soon as possible after symptom onset.4 Recurrent episodes are usually self-limited and antiviral therapy may not be required for patients with minimal or mild symptoms. For patients with frequent (i.e., episodes at least every 2 mo or at least 6 times/yr) or severe recurrences, daily suppressive antiviral therapy can be considered and should be re-evaluated annually.4
First-line treatment of genital herpes simplex virus infection*
Hypertrophic HSV infection is an atypical and uncommon manifestation of HSV. Described cases have most commonly involved the anogenital structures. In a review of 110 cases, 76.4% were anogenital; lesions of the oropharynx, nose, ears and ocular structures have also been reported.5 The clinical course is chronic and can be disfiguring, and the appearance is often mistaken for cutaneous malignant disease. Hypertrophic HSV infection has predominantly been described among people living with HIV infection. An association with immune reconstitution inflammatory syndrome has been hypothesized, although many cases have been described in patients on stable antiretroviral therapy with long-term virologic suppression.6 Anogenital involvement is most commonly seen among people living with HIV infection and may be related to sexual practices in MSM. Cases have also been seen with other forms of cellular immunodeficiency, such as hematologic malignancy, solid organ transplantation and congenital immune deficiencies (including common variable immunodeficiency secondary to T-cell lymphopenia, congenital T-cell deficiency syndrome and hyperimmunoglobulin E syndrome related to STAT3 sequence variations).5,6
The pathogenesis of hypertrophic HSV infection is poorly understood, although it may reflect chronic viral lytic replication in a host with underlying immune dysfunction.6 Our patient was taking ruxolitinib, a Janus kinase inhibitor, which is used for the treatment of myeloproliferative disorders including myelofibrosis. Increased frequency of Herpesviridae infections have been attributed to ruxolitinib, although these are most commonly varicella zoster virus infections rather than HSV.7 Diagnosis is generally made with biopsy of the lesion for histopathologic examination, HSV PCR or viral culture.5 Previous case reports have suggested that hypertrophic HSV infection is poorly responsive to conventional treatment with antiviral therapy.6 Alternate nonantiviral treatment modalities include surgical resection, topical imiquimod and thalidomide.5
Our patient’s management was complicated by resistance to acyclovir and related compounds via sequence variations in the UL23 thymidine kinase gene. Acyclovir and related compounds are the mainstay of treatment for HSV infections. They exert their effects through termination of viral DNA transcription.8 Upon entry into host cells, these antiviral agents undergo 3 consecutive phosphorylation reactions with conversion to acyclovir triphosphate, the active form.8 The first phosphorylation reaction is by viral thymidine kinase, while the second and third phosphorylation reactions are by host cell enzymes.8 Resistance to these compounds is primarily seen in immunocompromised hosts, such as people living with HIV infection and recipients of solid organ transplants.9 It is most often related to previous substantial exposure to acyclovir and related compounds.9 Our patient was immunocompromised owing to myelofibrosis and treatment with ruxolitinib, and had exposure to multiple courses of antiviral therapy over the previous 3 years, increasing his risk of resistance. Resistance mediated by sequence variations in the UL23 thymidine kinase gene results in absent, low production or altered activity of viral thymidine kinase, thereby preventing the first phosphorylation reaction. Less commonly, variations in the UL30 DNA polymerase gene result in target site alteration.8 In Canada, resistance genotyping is performed by Sanger sequencing at the National Microbiology Laboratory in Winnipeg.10 Alternate antiviral agents that can be used for resistant HSV include foscarnet and cidofovir; we prescribed the former for our patient. Foscarnet and cidofovir are inhibitors of viral DNA polymerase, but unlike acyclovir and related compounds, do not require phosphorylation by viral thymidine kinase. Both agents are administered intravenously and are associated with substantial risk of nephrotoxicity. Patients should be closely monitored for renal impairment and electrolyte disturbances; aggressive hydration and electrolyte replacement may be required.
We report a case of hypertrophic HSV infection in a man with myelofibrosis and substantial previous exposure to antiviral treatment, which was resistant to treatment with acyclovir and related compounds. Hypertrophic HSV infection is uncommon but can be seen in patients who are immunocompromised, most commonly people living with HIV infection. Resistance to antiviral agents should be suspected in patients who do not respond to conventional treatment, especially in patients who are immunocompromised or those with repeated antiviral exposure.
The section Cases presents brief case reports that convey clear, practical lessons. Preference is given to common presentations of important rare conditions, and important unusual presentations of common problems. Articles start with a case presentation (500 words maximum), and a discussion of the underlying condition follows (1000 words maximum). Visual elements (e.g., tables of the differential diagnosis, clinical features or diagnostic approach) are encouraged. Consent from patients for publication of their story is a necessity. See information for authors at www.cmaj.ca.
Footnotes
-
Competing interests: None declared.
-
This article has been peer reviewed.
-
The authors have obtained patient consent.
-
Contributors: Charlie Tan and Wayne Gold led the conception and design of the work. Charlie Tan wrote the first draft of the manuscript. All authors revised the manuscript critically for important intellectual content, approved the final version to be published and agree to be accountable for all aspects of the work.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/
Hypertrophic herpes simplex virus 2 infection resistant to acyclovir in an immunosuppressed patient - CMAJ
Read More

No comments:
Post a Comment