Abstract
Mild cognitive impairment (MCI) is the early stage of cognitive impairment between the expected cognitive decline of normal aging and the more serious decline of dementia. This meta-analysis and systematic review explored the pooled global prevalence of MCI among older adults living in nursing homes and its relevant factors. The review protocol was registered in INPLASY (INPLASY202250098). PubMed, Web of Science, Embase, PsycINFO, and CINAHL databases were systematically searched from their respective inception dates to 8 January 2022. The inclusion criteria were made based on the PICOS acronym, as follows: Participants (P): Older adults living in nursing homes; Intervention (I): not applicable; Comparison (C): not applicable; Outcome (O): prevalence of MCI or the data can generate the prevalence of MCI according to study-defined criteria; Study design (S): cohort studies (only baseline data were extracted) and cross-sectional studies with accessible data published in a peer-reviewed journal. Studies involving mixed resources, reviews, systematic reviews, meta-analyses, case studies, and commentaries were excluded. Data analyses were performed using Stata Version 15.0. Random effects model was used to synthesize the overall prevalence of MCI. An 8-item instrument for epidemiological studies was used to assess the quality of included studies. A total of 53 articles were included involving 376,039 participants with a mean age ranging from 64.42 to 86.90 years from 17 countries. The pooled prevalence of MCI in older adults in nursing homes was 21.2% (95% CI: 18.7–23.6%). Subgroup and meta-regression analyses revealed that the screening tools used were significantly associated with MCI prevalence. Studies using the Montreal Cognitive Assessment (49.8%) had a higher prevalence of MCI than those using other instruments. No significant publication bias was found. Several limitations warrant attention in this study; for example, significant heterogeneity between studies remained and some factors associated with the prevalence of MCI were not examined due to insufficient data. Adequate screening measures and allocation of resources are needed to address the high global prevalence of MCI among older adults living in nursing homes.
Introduction
Mild cognitive impairment (MCI) is often defined as complaints of memory deficits and abnormal memory function that differ from healthy age-matched individuals, normal general cognitive function, and activities of daily living, which, however, does not meet the criteria of dementia [1,2,3]. It may be a precursor of dementia, being a transitional state from normal aging to dementia. A previous study found that over 60% of people with MCI went on to develop clinical dementia during their life [4]. The conversion rate varied among different studies with an average annual rate of 10–15% [2, 5,6,7,8], and after 6 years over 80% developed dementia [4]. A meta-analysis found that the proportion of those with MCI who progressed to dementia was 39.2% in clinical settings such as memory clinics or hospitals, while the corresponding figure was 21.9% in community populations [9]. Another survey reported that individuals with MCI converted to probable dementia at a high-rate of 241.3/1,000 person-years (PY), which was almost four times the risk of those with normal cognition [10]. In addition, some studies suggested that participants with MCI had increased mortality compared to those with normal cognition [11,12,13].
Nursing homes are facilities for people who cannot be cared for at home but do not need to be in a hospital. They often provide a family-style environment with 24-h functional support and care for older people who need help with activities of daily living, have complex health care needs, and are more vulnerable [14]. Impaired cognitive function is one of the major contributing factors leading to the placement of older people in nursing homes [15]. For instance, a previous study found that mild to moderate cognitive impairment was associated with more than 7 times higher risk of nursing home admission and more than 5 times higher risk of death [16] than those without cognitive impairment. Nursing homes are a suitable choice to care for older people with increased severity of cognitive impairment as the professional care provided can improve their quality of life and alleviate the burden on family caregivers [17]. Epidemiological studies of MCI in those living in nursing homes provide a good basis to allocate sufficient health resources to provide early identification, prevention and timely treatment of MCI before it develops into dementia [18]. Studies that examined the prevalence of MCI among older adults living in nursing homes found mixed results ranging from 4.0% to 87.4% [18,19,20]. Further, most meta-analyses of the prevalence of MCI focused only on community-dwelling populations [10, 21,22,23]. For example, a meta-analysis of the overall prevalence of MCI reported a prevalence of 17.3% in community-dwelling older people [24]. Considering that the prevalence of MCI in those living in nursing homes appeared higher than that in the community [25, 26], the epidemiological findings obtained in the community could not be generalized to nursing home residents. To date, no meta-analysis or systematic review on the prevalence of MCI among older adults living in nursing homes has been published.
To fill in this gap, this meta-analysis examined the pooled global prevalence of MCI among older adults living in nursing homes and its associated factors.
Methods
Search strategy
This meta-analysis was conducted based on the guidelines of Meta-Analysis Of Observational Studies in Epidemiology (MOOSE) [27] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [28]. The registration number of this protocol was INPLASY202250098. A comprehensive literature search was conducted by two researchers (PC and HC) independently in major international databases from their inception dates to 8 January 2022, including PubMed, Web of Science, Embase, PsycINFO, and CINAHL. Search terms were as follows: (“cognitive dysfunction”[MeSH Terms] OR “mild cognitive impairment” or “MCI”) AND (“Nursing Homes” OR “Nursing Home” OR “Intermediate Care Facilities” OR “Intermediate Care Facility” OR “Skilled Nursing Facilities” OR “Skilled Nursing Facility” OR “Extended Care Facilities” OR “Extended Care Facility” OR “convalescence home” OR “convalescence hospital” OR “long-term care” OR “old age homes” OR “residential homes” OR “nursing home*” OR “residential care” OR “institutionalization*” OR “nursing home placement*” OR “nursing home admission*” OR “Homes, Nursing”) AND (“aged” OR “old age” OR “elderly” OR “late-life” OR “geriatric*” OR “older adult” OR “elder*”) AND (“prevalence” OR “epidemiology” OR “rate”). The search strategy is shown in Supplementary Table S1. The reference lists of relevant reviews [22, 29,30,31] were also searched manually for additional studies.
Inclusion and exclusion criteria
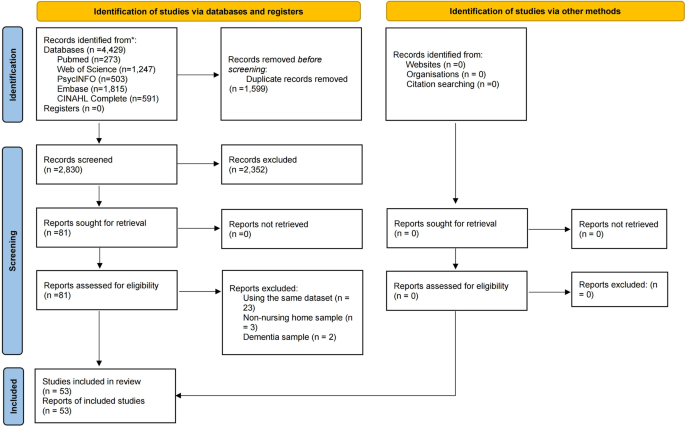
The same two researchers independently screened the titles and abstracts of publications and then read the full texts of the relevant publications to identify eligible studies. The inclusion criteria were made based on the PICOS acronym, as follows: Participants (P): Older adults living in nursing homes; Intervention (I): not applicable; Comparison (C): not applicable; Outcome (O): prevalence of MCI or the data can generate the prevalence of MCI according to study-defined criteria; Study design (S): cohort studies (only baseline data were extracted) and cross-sectional studies with accessible data published in a peer-reviewed journal. Studies involving mixed resources (e.g., nursing homes and communities), reviews, systematic reviews, meta-analyses, case studies, and commentaries were excluded. When multiple studies based on the same dataset were published, only the one with the largest sample size was included. Any discrepancies in the above procedures were resolved by a discussion with a third investigator (YTX). The process of study selection is shown in Fig. 1.
Data extraction and study quality assessment
Data were extracted independently by two investigators (PC and HC), including study characteristics (first author, publication year, survey time, countries, study design, sampling methods, and screening tool used for MCI) and sample characteristics (sample size, mean age, proportion of males, and number of participants with MCI). An 8-item instrument for epidemiological studies [32, 33] was used to assess the quality of included studies, including: (1) Target population was defined clearly; (2) Probability sampling or entire population surveyed; (3) Response rate was equal or greater than 80%; (4) Non-responders were clearly described; (5) Sample was representative of the target population; (6) Data collection methods was standardized; (7) Validated criteria were used to diagnose MCI; and (8) Prevalence estimates were given with confidence intervals and detailed by subgroups (if applicable). The total score ranges from 0 to 8, with low (0–3), moderate (4–6), and high (7–8) quality levels [34]. Disagreements between investigators in study assessments were resolved by a discussion with a third investigator (YTX).
Statistical analysis
The meta-analysis was performed by Stata version 15 software. Due to different demographic data and methodology (e.g., sampling method) between the studies, the pooled prevalence of MCI and 95% confidence intervals (CIs) were calculated using a random-effects model [35]. Cochran’s Q test and I2 statistics were used to quantify the heterogeneity across studies, the P < 0.1 or I2 > 50% was defined as significantly high heterogeneity [36]. Subgroup analyses for categorical variables (study regions, countries by economic status according to the World Bank’s criteria [37], sampling method, scales on MCI, age group, and survey year) and meta-regression analysis for continuous variables (mean age, male proportion, and quality assessment score) were used to explore the sources of potential heterogeneity. Sensitivity analyses were performed to evaluate the stability of results by excluding each study, one by one. Begg’s funnel plot and Egger’s tests were used to assess the publication bias of the included studies. A P < 0.05 (two-tailed) was considered statistically significant.
Result
Characteristics of the studies
In total, 4429 relevant publications were searched from the databases. After removing 1599 duplicate records, 2830 titles and abstracts were screened and the full text of 81 publications were reviewed for eligibility. Of them, 28 were excluded due to overlapping data based on the same dataset (n = 23), non-nursing home samples (n = 3) and dementia samples (n = 2). Finally, 53 eligible studies were included in this meta-analysis. In total, 376,039 participants with a mean age ranging from 64.42 to 86.90 years from 17 countries were included. Most of the studies were conducted in Europe & Central Asia (29; 54.7%), followed by North America (14; 26.4%), East Asia & Pacific (8; 15.1%), Middle East & North Africa (1; 1.9%), and Sub-Saharan Africa (1; 1.9%). The survey years ranged from 1982 to 2019. Nearly four-fifths of the studies were cross-sectional (42; 79.2%) and more than half (31; 58.5%) used convenience sampling. Of the 13 MCI screening measures, the Mini-Mental State Examination (MMSE) (24; 45.3%) was the most frequently used tool. Study quality assessment scores ranged from 4 to 7; 48 (90.6%) were considered “moderate” quality and 5 (9.4%) were considered “high” quality. Detailed characteristics and quality assessment scores are presented in Table 1 and Supplementary Table S2.
Prevalence of mild cognitive impairment
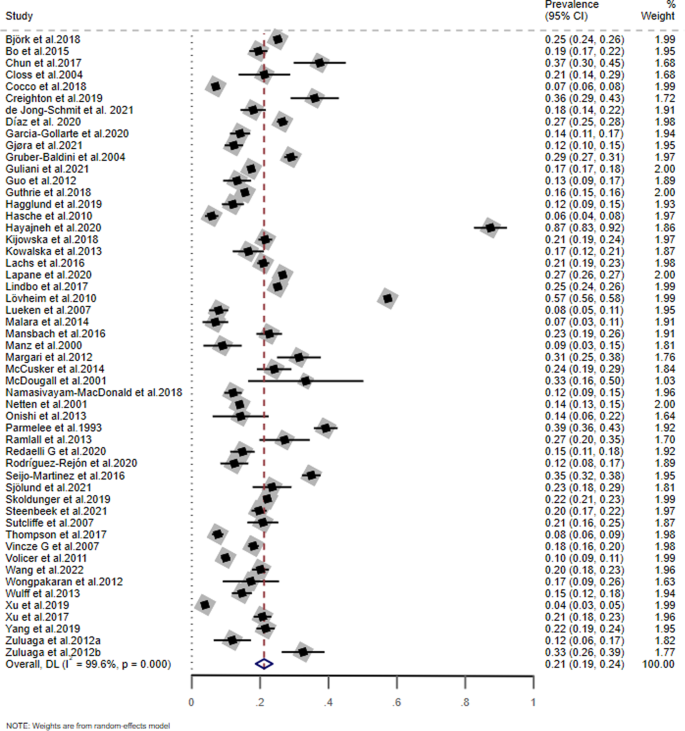
As shown in Fig. 2, the pooled prevalence of MCI based on the 53 included studies was 21.2% (95% CI: 18.7–23.6%; I2 = 99.6%).
Subgroup and meta-regression analyses
Table 2 presents the results of subgroup analyses. The screening tools used for MCI (Q = 16.51, P = 0.011) were significantly associated with the prevalence of MCI. Studies using the Montreal Cognitive Assessment (MoCA) (49.8%, 95% CI: 0–123.4%) had a higher prevalence of MCI than those using other instruments. For the meta-regression analyses, there were no significant associations between the prevalence of MCI and mean age (t = 0.54, P = 0.591), male proportion (t = −0.97, P = 0.340), and quality assessment score (t = 0.13, P = 0.900; Figs. S1–3).
Sensitivity analysis and publication bias
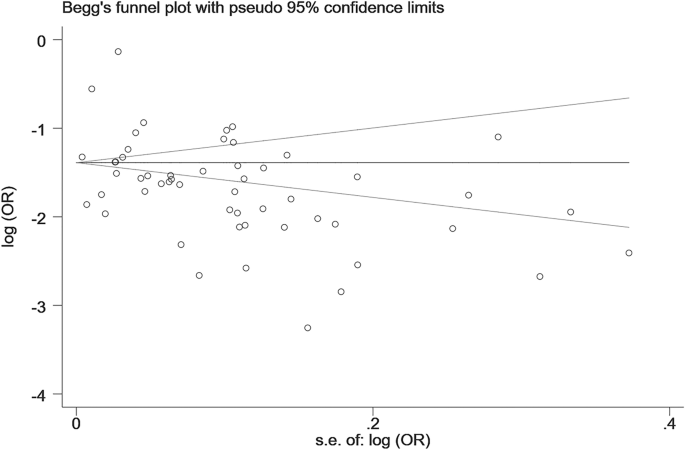
The results of sensitivity analyses are shown in Fig. S4. We did not find any outlying studies that could significantly affect the primary results. The Begg’s funnel plot (Begg’s test: z = −0.92, P = 0.357) and Egger’s test (t = −0.93; P = 0.358) did not find any significant publication biases (Fig. 3).
Discussion
To the best of our knowledge, this was the first meta-analysis to estimate the pooled global prevalence of MCI among older adults living in nursing homes. This meta-analysis included 53 studies across 17 countries and found an overall prevalence of 21.2% (95% CI: 18.7–23.6%; I2 = 99.6%), which is higher than the findings in the general community populations (17.3%; 95% CI: 13.8–20.8%) [24] and Chinese community-dwelling populations (12.2%; 95% CI: 10.6–14.2%) [22]. There are several reasons for this discrepancy. Cognitive impairment is one of the major reasons for admission to nursing homes [25, 38], which would result in a higher rate of MCI in nursing homes residents compared to community-dwelling population. In addition, other key reasons for nursing home placement include major physical and psychiatric disorders, such as physical pain [39], diabetes [40], depression [41], and anxiety [42], all of which could be associated with a higher risk of cognitive impairment [43, 44].
MCI is a pathological condition that encompasses a series of symptoms related to cognition rather than being defined as a disease [45]. It often evolves gradually into memory loss and difficulty in communication and handling complex tasks, visual and spatial abilities, planning and organization, coordination and motor functions, and disorientation [46]. Thus, early identification is crucial to prevent the deterioration of cognition impairment [4]. Despite the high conversion rate to dementia, there remains a small proportion of persons with MCI who can recover to a normal cognitive level [10], which highlights the importance of early management of MCI. Certain interventional measures for MCI, such as cognitive training [47], physical exercise [48], and diet regulation [48], appeared to have some symptomatic benefits although there is no effective pharmacological treatment for MCI [45, 49]. The guidelines for the management of MCI propose a multi-targeted treatment approach [45], which includes a range of strategies to improve cognitive performance in this population.
Various scales are used to screen MCI such as the MoCA, Mini-Mental State Examination (MMSE), Cognitive Performance Scale (CPS), Gottfries cognitive scale (GCS), Pfeiffer test,) and Short Portable Mental Status Questionnaire (SPSMQ). In this study, subgroup analyses revealed a significant difference in MCI prevalence between different MCI screening tools used, with those using the MoCA having the highest prevalence (49.8%). MoCA is a brief cognitive screening tool with excellent sensitivity (90%) and specificity (87%) [50, 51], which covers short-term memory, visuospatial skills, executive function, attention, concentration and working memory, language, and orientation. The MoCA assessment requires 10–20 min to complete, which is influenced by the education level of the participant, hence, an extra point is added to the MoCA total score for participants with <13 years of education [52]. The MMSE is another widely used tool in screening cognition levels, with acceptable sensitivity (13–97%) and specificity (60–100%) across different studies [53,54,55], that covers multiple domains, including orientation, attention and calculation, language, immediate recall, short-term memory, registration, and construct ability. The MMSE assessment needs 5–10 min to complete, which is also associated with the education level of the patient [52]. However, the MMSE is not a reliable test for detecting MCI at an early stage [53]. Sensitivity, specificity, and time efficiency are the main factors in evaluating such screening tools [52], therefore, various screening tools could contribute to different results for MCI prevalence [56]. Previous studies found that the MoCA showed better specificity and sensitivity in detecting MCI than other cognitive measures such as the MMSE [50, 53, 54]. However, it should be noted that as only two studies using the MoCA were included in our meta-analysis, this finding may be preliminary and needs to be confirmed in future studies. The CPS, which is similar to the MMSE in identifying cognitive impairment, was initially applied to nursing home residents with good sensitivity (87–94%) and specificity (80–95%) [57]. Although the CPS assessment is not influenced by age and education level, it requires more than 30 min for completion [52]. Overall, the assessment duration and the potential influence of education level on the results should be considered in selecting a suitable screening instrument for MCI.
Older age is a risk factor for cognitive decline and could accelerate the progression of cognitive impairment [56, 58]. The findings on the relationship between age and MCI prevalence were mixed. Some studies did not find a significant association [8, 59], while others showed a significant association between age and MCI prevalence, with a higher prevalence in older individuals [56, 60,61,62]. In this meta-analysis, the pooled prevalence of MCI was 18.2%, 15.0%, and 21.7% in the 70–74-, 75–79-, and 80–84-years age groups, respectively, but the difference between age groups did not reach a significant level.
Similarly, the association between gender and MCI prevalence is also controversial. For instance, some studies did not find a significant association between gender and MCI prevalence [56, 60, 62], while other studies reported that either males [8, 61] or females had a higher prevalence of MCI [59]. One study attributed the possible reason for higher MCI prevalence in males to the higher proportion of males in the study sample [8]. The higher prevalence of MCI in females may be due to hormonal differences between males and females [22]; i.e., estrogen exposure plays a role in brain aging, which is associated with changes of global cognitive functioning and verbal attention [63]. The decreased estrogen levels in females after menopause can lead to partial impairment of cognitive function such as verbal memory, reasoning, and vigilance [64]. In this meta-analysis, however, there were no significant gender difference in terms of MCI prevalence.
We also did not find significant differences in the prevalence of MCI among older adults in nursing homes between geographical regions and between different income levels, which are not consistent with the findings in the community-dwelling older populations [65]. The pooled prevalence of MCI among older people living in nursing homes was 19.7% in Europe & Central Asia, 20.7% in North America, and 18.2% in East Asia & Pacific in this study, while the corresponding rate was 10.9%, 15.5% and 19.0%, respectively among community-dwelling older populations [65]. We speculate that compared to those living in the community, older adults living in nursing homes usually received better support and health care, which could offset the differences of MCI prevalence caused by different regions and economic factors.
The strengths of this meta-analysis included a large number of studies, the use of sophisticated analyses (e.g., subgroup and meta-regression analyses) and the homogeneous study sample of nursing homes residents. However, several limitations warrant attention in this study. Firstly, significant heterogeneity between studies remained even when subgroup analyses were performed, since heterogeneity is a common phenomenon in the meta-analysis of epidemiological surveys [66]. Second, some factors associated with the prevalence of MCI, such as education level, economic status, marital status, and MCI subtypes, were not examined due to insufficient data. Third, MCI was assessed using self-report scales in most studies, rather than diagnostic clinical interviews. MCI prevalence among those living in nursing homes may be better examined based on standard diagnostic criteria such as the Petersen’s criteria [2].
In summary, this meta-analysis showed that the global prevalence of MCI was over 20% among older adults living in nursing homes. Adequate screening measures and allocation of resources are needed to address the high global prevalence of MCI among older adults living in nursing homes. Early identification, preventive interventions and dementia treatment and care are essential to reduce the health burden of MCI in this population.
References
-
Etgen T, Sander D, Bickel H, Förstl H. Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch Ärzteblatt Int. 2011;108:743.
-
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. https://doi.org/10.1001/archneur.58.12.1985
-
Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, memory, and mild cognitive impairment. Int Psychogeriatr. 1997;9:65–9. https://doi.org/10.1017/s1041610297004717
-
Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. Br J Psychiatry. 2006;189:399–404. https://doi.org/10.1192/bjp.bp.105.014779
-
Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16:129–40. https://doi.org/10.1017/s1041610204000092
-
DeCarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2:15–21. https://doi.org/10.1016/s1474-4422(03)00262-x
-
Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151–7. https://doi.org/10.1001/archneurol.2009.106
-
Guo M, Gao L, Zhang G, Li Y, Xu S, Wang Z, et al. Prevalence of dementia and mild cognitive impairment in the elderly living in nursing and veteran care homes in Xi’an, China. J Neurol Sci. 2012;312:39–44. https://doi.org/10.1016/j.jns.2011.08.026
-
Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–65. https://doi.org/10.1111/j.1600-0447.2008.01326.x
-
Zhang Y, Natale G, Clouston S. Incidence of mild cognitive impairment, conversion to probable dementia, and mortality. Am J Alzheimers Dis Other Demen. 2021;36:15333175211012235 https://doi.org/10.1177/15333175211012235
-
Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–72. https://doi.org/10.1016/j.cger.2013.07.003
-
Wasserman D, Carli V, Iosue M, Javed A, Herrman H. Suicide prevention in psychiatric patients. Asia-Pac Psychiatry. 2021;13:e12450 https://doi.org/10.1111/appy.12450
-
de Mendonça Lima CA, De Leo D, Ivbijaro G, Svab I. Suicide prevention in older adults. Asia-Pac Psychiatry. 2021;13:e12473 https://doi.org/10.1111/appy.12473
-
Sanford AM, Orrell M, Tolson D, Abbatecola AM, Arai H, Bauer JM, et al. An international definition for “Nursing Home”. J Am Med Dir Assoc. 2015;16:181–4. https://doi.org/10.1016/j.jamda.2014.12.013
-
Dramé M, Lang P-O, Jolly D, Narbey D, Mahmoudi R, Lanièce I, et al. Nursing home admission in elderly subjects with dementia: predictive factors and future challenges. J Am Med Dir Assoc. 2012;13:83.e17–20. https://doi.org/10.1016/j.jamda.2011.03.002
-
Schaller F, Sidelnikov E, Theiler R, Egli A, Staehelin HB, Dick W, et al. Mild to moderate cognitive impairment is a major risk factor for mortality and nursing home admission in the first year after hip fracture. Bone. 2012;51:347–52. https://doi.org/10.1016/j.bone.2012.06.004
-
Gaugler JE, Yu F, Davila HW, Shippee T. Alzheimer’s disease and nursing homes. Health Aff (Millwood). 2014;33:650–7. https://doi.org/10.1377/hlthaff.2013.1268
-
Hayajneh AA, Rababa M, Alghwiri AA, Masha’al D. Factors influencing the deterioration from cognitive decline of normal aging to dementia among nursing home residents. BMC Geriatr. 2020;20:479 https://doi.org/10.1186/s12877-020-01875-3
-
Jeong A, Lapenskie J, Talarico R, Hsu AT, Tanuseputro P. Health outcomes of immigrants in nursing homes: a population-based retrospective cohort study in Ontario, Canada. J Am Med Dir Assoc. 2020;21:740–6.e745. https://doi.org/10.1016/j.jamda.2020.03.001
-
Xu, R, Zhou, X, Cao, S, Huang, B, Wu, C, Zhou, X et al. Health status of the elderly and its influence on their activities of daily living in Shangrao, Jiangxi province. Int J Environ Res Public Health. 2019. https://doi.org/10.3390/ijerph16101771.
-
Brodaty H, Heffernan M, Kochan NA, Draper B, Trollor JN, Reppermund S, et al. Mild cognitive impairment in a community sample: The Sydney Memory and Ageing Study. Alzheimer’s Dement. 2013;9:310–7.e311. https://doi.org/10.1016/j.jalz.2011.11.010
-
Lu Y, Liu C, Yu D, Fawkes S, Ma J, Zhang M, et al. Prevalence of mild cognitive impairment in community-dwelling Chinese populations aged over 55 years: a meta-analysis and systematic review. BMC Geriatr. 2021;21:10. https://doi.org/10.1186/s12877-020-01948-3
-
Ng TKS, Feng L, Chua RY, Goh LG, Kua EH, Mahendran R. A 5-year community program in Singapore to prevent cognitive decline. Asia-Pac Psychiatry. 2022;14:e12518. https://doi.org/10.1111/appy.12518
-
Pessoa RMP, Bomfim AJL, Ferreira BLC, Chagas MHN. Diagnostic criteria and prevalence of mild cognitive impairment in older adults living in the community: a systematic review and meta-analysis. Arch Clin Psychiatry (São Paulo). 2019;46:72–9.
-
Kijowska V, Szczerbińska K. Prevalence of cognitive impairment among long-term care residents: a comparison between nursing homes and residential homes in Poland. Eur Geriatr Med. 2018;9:467–76. https://doi.org/10.1007/s41999-018-0062-2
-
Mansbach WE, Mace RA, Clark KM. Mild cognitive impairment (MCI) in long-term care patients: Subtype classification and occurrence. Aging Ment Health. 2016;20:271–6. https://doi.org/10.1080/13607863.2014.1003283
-
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Jama. 2000;283:2008–12.
-
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:1–11.
-
Deng Y, Zhao S, Cheng G, Yang J, Li B, Xu K, et al. The prevalence of mild cognitive impairment among chinese people: a meta-analysis. Neuroepidemiology. 2021;55:79–91. https://doi.org/10.1159/000512597
-
Alexander M, Perera G, Ford L, Arrighi HM, Foskett N, Debove C, et al. Age-stratified prevalence of mild cognitive impairment and dementia in European populations: a systematic review. J Alzheimer’s Dis. 2015;48:355–9.
-
Au B, Dale-McGrath S, Tierney MC. Sex differences in the prevalence and incidence of mild cognitive impairment: a meta-analysis. Ageing Res Rev. 2017;35:176–99. https://doi.org/10.1016/j.arr.2016.09.005
-
Boyle MH. Guidelines for evaluating prevalence studies. Evid-based Ment Health. 1998;1:37–9.
-
Loney PL, Chambers LW, Bennett KJ, Roberts JG, Stratford PW. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19:170–6.
-
Yang C, Zhang L, Zhu P, Zhu C, Guo Q. The prevalence of tic disorders for children in China: a systematic review and meta-analysis. Medicine. 2016;95:e4354.
-
Harris RJ, Deeks JJ, Altman DG, Bradburn MJ, Harbord RM, Sterne JAC. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8:3–28. https://doi.org/10.1177/1536867X0800800102
-
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557
-
The World Bank Group. Countries and Economies. https://data.worldbank.org/ (2022).
-
Helvik AS, Skancke RH, Selbæk G, Engedal K. Nursing home admission during the first year after hospitalization - the contribution of cognitive impairment. PLoS ONE. 2014;9:e86116. https://doi.org/10.1371/journal.pone.0086116
-
Björk S, Juthberg C, Lindkvist M, Wimo A, Sandman P-O, Winblad B, et al. Exploring the prevalence and variance of cognitive impairment, pain, neuropsychiatric symptoms and ADL dependency among persons living in nursing homes; a cross-sectional study. BMC Geriatr. 2016;17:1–8. https://doi.org/10.1186/s12877-016-0328-9
-
Bo M, Gallo S, Zanocchi M, Maina P, Balcet L, Bonetto M. et al. Prevalence, clinical correlates, and use of glucose-lowering drugs among older patients with type 2 diabetes living in long-term care facilities. J Diabetes Res. 2015. https://doi.org/10.1155/2015/174316.
-
McCusker J, Cole MG, Voyer P, Monette J, Champoux N, Ciampi A, et al. Observer-rated depression in long-term care: Frequency and risk factors. Arch Gerontol Geriatr. 2014;58:332–8. https://doi.org/10.1016/j.archger.2013.11.010
-
Creighton AS, Davison TE, Kissane DW. The prevalence, reporting, and treatment of anxiety among older adults in nursing homes and other residential aged care facilities. J Affect Disord. 2018;227:416–23. https://doi.org/10.1016/j.jad.2017.11.029
-
Tangalos EG, Petersen RC. Mild cognitive impairment in geriatrics. Clin Geriatr Med. 2018;34:563–89. https://doi.org/10.1016/j.cger.2018.06.005
-
Yeo JJ, Chew QH, Sim K. Resilience and its inter-relationship with symptomatology, illness course, psychosocial functioning, and mediational roles in schizophrenia: a systematic review. Asia-Pac Psychiatry. 2022;14:e12486. https://doi.org/10.1111/appy.12486
-
Kasper S, Bancher C, Eckert A, Förstl H, Frölich L, Hort J, et al. Management of mild cognitive impairment (MCI): The need for national and international guidelines. World J Biol Psychiatry. 2020;21:579–94. https://doi.org/10.1080/15622975.2019.1696473
-
Gale SA, Acar D, Daffner KR. Dementia. Am J Med. 2018;131:1161–9. https://doi.org/10.1016/j.amjmed.2018.01.022
-
Horr T, Messinger-Rapport B, Pillai JA. Systematic review of strengths and limitations of randomized controlled trials for non-pharmacological interventions in mild cognitive impairment: focus on Alzheimer’s disease. J Nutr Health Aging. 2015;19:141–53. https://doi.org/10.1007/s12603-014-0565-6
-
Ströhle A, Schmidt DK, Schultz F, Fricke N, Staden T, Hellweg R, et al. Drug and exercise treatment of alzheimer disease and mild cognitive impairment: a systematic review and meta-analysis of effects on cognition in randomized controlled trials. Am J Geriatr Psychiatry. 2015;23:1234–49. https://doi.org/10.1016/j.jagp.2015.07.007
-
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:126–35. https://doi.org/10.1212/wnl.0000000000004826
-
Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. https://doi.org/10.1111/j.1532-5415.2005.53221.x
-
Abd Razak MA, Ahmad NA, Chan YY, Mohamad Kasim N, Yusof M, Abdul Ghani MKA, et al. Validity of screening tools for dementia and mild cognitive impairment among the elderly in primary health care: a systematic review. Public Health. 2019;169:84–92. https://doi.org/10.1016/j.puhe.2019.01.001
-
Zhuang L, Yang Y, Gao J. Cognitive assessment tools for mild cognitive impairment screening. J Neurol. 2021;268:1615–22. https://doi.org/10.1007/s00415-019-09506-7
-
Ozer S, Young J, Champ C, Burke M. A systematic review of the diagnostic test accuracy of brief cognitive tests to detect amnestic mild cognitive impairment. Int J Geriatr Psychiatry. 2016;31:1139–50. https://doi.org/10.1002/gps.4444
-
Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kędziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50:1039–52.
-
Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Júnior AL, Costa MLG, Ximenes RCC, et al. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int Psychogeriatr. 2019;31:491–504. https://doi.org/10.1017/s1041610218001370
-
Ramlall S, Chipps J, Pillay BJ, Bhigjee AI. Mild cognitive impairment and dementia in a heterogeneous elderly population: prevalence and risk profile. Afr J Psychiatry. 2013;16:456–65. https://doi.org/10.4314/ajpsy.v16i6.58
-
Morris JN, Howard EP, Steel K, Perlman C, Fries BE, Garms-Homolová V, et al. Updating the Cognitive Performance Scale. J Geriatr Psychiatry Neurol. 2015;29:47–55. https://doi.org/10.1177/0891988715598231
-
Bickel H, Hendlmeier I, Heßler JB, Junge MN, Leonhardt-Achilles S, Weber J, et al. The prevalence of dementia and cognitive impairment in hospitals. Dtsch Arztebl Int. 2018;115:733–40. https://doi.org/10.3238/arztebl.2018.0733
-
Björk S, Lövheim H, Lindkvist M, Wimo A, Edvardsson D. Thriving in relation to cognitive impairment and neuropsychiatric symptoms in Swedish nursing home residents. Int J Geriatr Psychiatry. 2018;33:e49–e57. https://doi.org/10.1002/gps.4714
-
Yang, L, Jin, XQ, Yan, J, Jin, Y, Xu, SH, Xu, Y et al. Comparison of prevalence and associated risk factors of cognitive function status among elderly between nursing homes and common communities of China A STROBE-compliant observational study. Medicine. 2019. https://doi.org/10.1097/md.0000000000018248.
-
Gjøra L, Strand BH, Bergh S, Borza T, Brækhus A, Engedal K, et al. Current and future prevalence estimates of mild cognitive impairment, dementia, and its subtypes in a population-based sample of people 70 years and older in Norway: The HUNT Study. J Alzheimer’s Dis. 2021;79:1213–26. https://doi.org/10.3233/JAD-201275
-
Vincze G, Álmos P, Boda K, Döme P, Bódi N, Szlávik G, et al. Risk factors of cognitive decline in residential care in Hungary. Int J Geriatr Psychiatry. 2007;22:1208–16. https://doi.org/10.1002/gps.1815
-
Smith CA, McCleary CA, Murdock GA, Wilshire TW, Buckwalter DK, Bretsky P, et al. Lifelong estrogen exposure and cognitive performance in elderly women. Brain Cogn. 1999;39:203–18. https://doi.org/10.1006/brcg.1999.1078
-
LeBlanc ES, Janowsky J, Chan BK, Nelson HD. Hormone replacement therapy and cognition: systematic review and meta-analysis. JAMA. 2001;285:1489–99.
-
Bai, W, Chen, P, Cai, H, Qinge, Z, Zhaohui, S, Teris, C et al. Worldwide prevalence of mild cognitive impairment among community dwellers aged 50 years and older: a meta-analysis and systematic review of epidemiology studies (in press). Age Ageing. 2022;51:afac173.
-
Cai H, Jin Y, Liu S, Zhang Q, Zhang L, Cheung T, et al. Prevalence of suicidal ideation and planning in patients with major depressive disorder: a meta-analysis of observation studies. J Affect Disord. 2021;293:148–158. https://doi.org/10.1016/j.jad.2021.05.115
-
Chun A, Reinhardt JP, Ramirez M, Ellis JM, Silver S, Burack O, et al. Depression recognition and capacity for self-report among ethnically diverse nursing homes residents: evidence of disparities in screening. J Clin Nurs. 2017;26:4915–26. https://doi.org/10.1111/jocn.13974
-
Closs SJ, Barr B, Briggs M. Cognitive status and analgesic provision in nursing home residents. Br J Gen Pract. 2004;54:919–21.
-
Cocco F, Campus G, Strohmenger L, Ardizzone VC, Cagetti MG. The burden of tooth loss in Italian elderly population living in nursing homes. BMC Geriatr. 2018;18:76 https://doi.org/10.1186/s12877-018-0760-0
-
Creighton AS, Davison TE, Kissane DW. The factors associated with anxiety symptom severity in older adults living in nursing homes and other residential aged care facilities. J Aging Health. 2019;31:1235–58. https://doi.org/10.1177/0898264318767781
-
de Jong-Schmit BEM, Poortvliet RKE, Bohringer S, Bogaerts JMK, Achterberg WP, Husebo BS. Blood pressure, antihypertensive medication and neuropsychiatric symptoms in older people with dementia: TheCOSMOSstudy. Int J Geriatr Psychiatry. 2021;36:46–53. https://doi.org/10.1002/gps.5388
-
Díaz LB, Casuso-Holgado MJ, Labajos-Manzanares MT, Barón-López FJ, Pinero-Pinto E, Romero-Galisteo RP, et al. Analysis of fall risk factors in an aging population living in long-term care institutions in Spain: A retrospective cohort study. Int J Environ Res Public Health. 2020;17:1–10. https://doi.org/10.3390/ijerph17197234
-
Garcia-Gollarte, JF, Garcia-Andrade, MM, Santaeugenia-Gonzalez, SJ, Hermida, JCS, Baixauli-Alacreu, S, Santabalbina, FJT. Risk factors for mortality in nursing home residents: an observational study. Geriatrics. 2020. https://doi.org/10.3390/geriatrics5040071.
-
Gruber-Baldini AL, Boustani M, Sloane PD, Zimmerman S. Behavioral symptoms in residential care/assisted living facilities: prevalence, risk factors, and medication management. J Am Geriatr Soc. 2004;52:1610–17. https://doi.org/10.1111/j.1532-5415.2004.52451.x
-
Guliani H, Hadjistavropoulos T, Jin S, Lix LM. Pain-related health care costs for long-term care residents. BMC Geriatr. 2021;21:552 https://doi.org/10.1186/s12877-021-02424-2
-
Guthrie, DM, Davidson, JGS, Williams, N, Campos, J, Hunter, K, Mick, P et al. Combined impairments in vision, hearing and cognition are associated with greater levels of functional and communication difficulties than cognitive impairment alone: Analysis of interRAI data for home care and long-term care recipients in Ontario. PLoS ONE. 2018. https://doi.org/10.1371/journal.pone.0192971.
-
Hagglund P, Koistinen S, Olai L, Stahlnacke K, Wester P, Jaghagen EL. Older people with swallowing dysfunction and poor oral health are at greater risk of early death. Community Dent Oral Epidemiol. 2019;47:494–501. https://doi.org/10.1111/cdoe.12491
-
Hasche LK, Morrow-Howell N, Proctor EK. Quality of life outcomes for depressed and nondepressed older adults in community long-term care. Am J Geriatr Psychiatry. 2010;18:544–53. https://doi.org/10.1097/JGP.0b013e3181cc037b
-
Kowalska J, Rymaszewska J, Szczepańska-Gieracha J. Occurrence of cognitive impairment and depressive symptoms among the elderly in a nursing home facility. Adv Clin Exp Med. 2013;22:111–7.
-
Lachs MS, Teresi JA, Ramirez M, van Haitsma K, Silver S, Eimicke JP, et al. The prevalence of resident-to-resident elder mistreatment in nursing homes. Ann Intern Med. 2016;165:229–36. https://doi.org/10.7326/M15-1209
-
Lapane KL, Hume AL, Morrison RA, Jesdale BM. Prescription analgesia and adjuvant use by pain severity at admission among nursing home residents with non-malignant pain. Eur J Clin Pharmacol. 2020;76:1021–8. https://doi.org/10.1007/s00228-020-02878-0
-
Lindbo A, Gustafsson M, Isaksson U, Sandman P-O, Lövheim H. Dysphoric symptoms in relation to other behavioral and psychological symptoms of dementia, among elderly in nursing homes. BMC Geriatr. 2017;17:1–8. https://doi.org/10.1186/s12877-017-0603-4
-
Lövheim H, Bergdahl E, Sandman PO, Karlsson S, Gustafson Y, Lövheim H, et al. One-week prevalence of depressive symptoms and psychotropic drug treatments among old people with different levels of cognitive impairment living in institutional care: changes between 1982 and 2000. Int Psychogeriatr. 2010;22:1154–60. https://doi.org/10.1017/S104161021000061X
-
Lueken U, Seidl U, Völker L, Schweiger E, Kruse A, Schröder J. Development of a short version of the Apathy Evaluation Scale specifically adapted for demented nursing home residents. Am J Geriatr Psychiatry. 2007;15:376–85. https://doi.org/10.1097/JGP.0b013e3180437db3
-
Malara A, Sgrò G, Caruso C, Ceravolo F, Curinga G, Renda GF, et al. Relationship between cognitive impairment and nutritional assessment on functional status in Calabrian long-term-care. Clin Inter Aging. 2014;9:105–10. https://doi.org/10.2147/cia.S54611
-
Manz BD, Mosier R, Nusser-Gerlach MA, Bergstrom N, Agrawal S. Pain assessment in the cognitively impaired and unimpaired elderly. Pain Manag Nurs. 2000;1:106–15.
-
Margari F, Sicolo M, Spinelli L, Mastroianni F, Pastore A, Craig F, et al. Aggressive behavior, cognitive impairment, and depressive symptoms in elderly subjects. Neuropsychiatr Dis Treat. 2012;8:347–53. https://doi.org/10.2147/ndt.S33745
-
McDougall GJ. Rehabilitation of memory and memory self-efficacy in cognitively impaired nursing home residents. Clin Gerontol. 2001;23:127–39. https://doi.org/10.1300/J018v23n03_11
-
Namasivayam-MacDonald AM, Slaughter SE, Morrison J, Steele CM, Carrier N, Lengyel C, et al. Inadequate fluid intake in long term care residents: prevalence and determinants. Geriatr Nurs. 2018;39:330–5. https://doi.org/10.1016/j.gerinurse.2017.11.004
-
Netten A, Darton R, Bebbington A, Forder J, Brown P, Mummery K. Residential and nursing home care of elderly people with cognitive impairment: Prevalence, mortality and costs. Aging Ment Health. 2001;5:14–22. https://doi.org/10.1080/13607860020020591
-
Onishi K. The challenge of estimating the prevalence of dementia in the elderly. Jpn Hosp. 2013;32:39–46.
-
Parmelee PA, Smith B, Katz IR. Pain complaints and cognitive status among elderly institution residents. J Am Geriatr Soc. 1993;41:517–22.
-
Redaelli G, Giunco F, Trimarchi PD, Carini F. Oral condition assessment among a nursing home population. Analysis of the association between tooth loss and cognitive impairment: An observational study. J Gerontol Geriatr. 2020;68:1–6. https://doi.org/10.36150/2499-6564-296
-
Rodríguez-Rejón AI, Artacho R, Ruiz-López MD. Anthropometric measurements and cognitive impairment rather than nutrition status are associated with sarcopenia in long-term care residents. Nutr Clin Pract. 2020;35:642–8. https://doi.org/10.1002/ncp.10370
-
Seijo-Martinez M, Cancela JM, Ayán C, Varela S, Vila H. Influence of cognitive impairment on fall risk among elderly nursing home residents. Int Psychogeriatr. 2016;28:1975–87. https://doi.org/10.1017/s1041610216001113
-
Sjölund B-M, Mamhidir A-G, Engström M. Pain prevalence among residents living in nursing homes and its association with quality of life and well-being. Scand J Caring Sci. 2021;35:1332–41. https://doi.org/10.1111/scs.12955
-
Skoldunger A, Wimo A, Sjogren K, Bjork S, Backman A, Sandman PO, et al. Resource use and its association to cognitive impairment, ADL functions, and behavior in residents of Swedish nursing homes: results from the U-Age program (SWENIS study). Int J Geriatr Psychiatry. 2019;34:130–6. https://doi.org/10.1002/gps.5000
-
Steenbeek ED, Ramspek CL, van Diepen M, Dekker FW, Achterberg WP. The association between pain perception and care dependency in older nursing home residents: a prospective cohort study. J Am Med Dir Assoc. 2021;22:676–81. https://doi.org/10.1016/j.jamda.2020.07.022
-
Sutcliffe C, Burns A, Challis D, Mozley CG, Cordingley L, Bagley H, et al. Depressed mood, cognitive impairment, and survival in older people admitted to care homes in england. Am J Geriatr Psychiatry. 2007;15:708–15. https://doi.org/10.1097/JGP.0b013e3180381537
-
Thompson GN, Doupe M, Reid RC, Baumbusch J, Estabrooks CA. Pain trajectories of nursing home residents nearing death. J Am Med Dir Assoc. 2017;18:700–6. https://doi.org/10.1016/j.jamda.2017.03.002
-
Volicer L, Frijters DHM, van der Steen JT. Underdiagnosis and undertreatment of depression in nursing home residents. Eur Geriatr Med. 2011;2:332–7. https://doi.org/10.1016/j.eurger.2011.08.001
-
Wang J, Liu W, Peng D, Xiao M, Zhao Q. The use of physical restraints in Chinese long-term care facilities and its risk factors: an observational and cross-sectional study. J Adv Nurs. 2020;76:2597–609. https://doi.org/10.1111/jan.14486
-
Wongpakaran N, Wongpakaran T. Prevalence of major depressive disorders and suicide in long‐term care facilities: a report from Northern Thailand. Psychogeriatrics. 2012;12:11–7. https://doi.org/10.1111/j.1479-8301.2011.00383.x
-
Wulff I, Kölzsch M, Kalinowski S, Kopke K, Fischer T, Kreutz R, et al. Perceived enactment of autonomy of nursing home residents: a German cross-sectional study. Nurs Health Sci. 2013;15:186–93. https://doi.org/10.1111/nhs.12016
-
Xu S, Jin X, Liu C, Jin Y, Xu Y, Chen L, et al. Investigating the prevalence of dementia and its associated risk factors in a chinese nursing home. J Clin Neurol. 2017;13:10–4. https://doi.org/10.3988/jcn.2017.13.1.10
-
Zuluaga DJ, Ferreira J, Montoya JA, Willumsen T. Oral health in institutionalised elderly people in Oslo, Norway and its relationship with dependence and cognitive impairment. Gerodontology. 2012a;29:e420–6.
-
Zuluaga DJM, Montoya JAG, Contreras CI, Herrera RR. Association between oral health, cognitive impairment and oral health-related quality of life. Gerodontology. 2012b;29:E667–73. https://doi.org/10.1111/j.1741-2358.2011.00542.x
Acknowledgements
This study was supported by the Scientific Research Common Program of Beijing Municipal Commission of Education (Grant No.: KM202010025011), the Beijing Municipal Science & Tech Commission (Grant No: Z191100006619061), the capital health research and development of special (Grant No.: 2022-3-2124), Beijing Talents Foundation (Grant No.: 2017000021469G222), and the University of Macau (MYRG2019-00066-FHS).
Author information
Authors and Affiliations
Contributions
Study design: PC, HC, WB, QZ, and Y-TX. Data collection, analysis and interpretation: PC, HC, ZS, and GSU. Drafting of the manuscript: PC, Y-LT, GSU, and Y-TX. Critical revision of the manuscript: CHN. Approval of the final version for publication: all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, P., Cai, H., Bai, W. et al. Global prevalence of mild cognitive impairment among older adults living in nursing homes: a meta-analysis and systematic review of epidemiological surveys. Transl Psychiatry 13, 88 (2023). https://ift.tt/Rl8EGL6
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
DOI: https://ift.tt/Rl8EGL6
Global prevalence of mild cognitive impairment among older adults living in nursing homes: a meta-analysis and systematic review of epidemiological surveys | Translational Psychiatry - Nature.com
Read More

No comments:
Post a Comment