In multicellular organisms, there are three types of protein glycosylation. N-glycosylation, O-mannosylation and C-mannosylation. All of these processes take place in the endoplasmic reticulum, and in all of them enzymes attach sugar residues to specific sites in newly forming protein.
While N- and O-glycosylation are well studied, the third form, C-mannosylation of tryptophan side chains, has long been a mystery to researchers. Although 20 percent of all secretory proteins, as well as membrane proteins, are affected by it, it was unclear until recently what the change was for, how the specific protein sequences are recognized and how the associated enzyme reaction is chemically possible at all.
In an international collaboration, researchers from ETH Zurich, the Walter and Eliza Hall Institute of Medical Research (WEHI) in Australia, the University of Chicago and the University of Bern have now elucidated the structure and function of the responsible enzyme, 'tryptophan C-mannosyltransferase' (CMT). The corresponding study was published in the latest issue of the journal Nature Chemical Biology.
CMT is a member of the category C (GT-C) glycosyltransferase enzymes, one of the three glycosyltransferase superfamilies. The most prominent member is oligosaccharyltransferase (OST), which is responsible for N-glycosylation.
Similar to the OST, the CMT also recognizes highly specific sequences in proteins, with the difference, however, that in mammals four different CMTs occur simultaneously, which also recognize different protein sequences.
Sugars help immuno-receptors to the cell surface
Only in recent years, the necessary tools, such as special antibodies and mass spectrometry test methods, were developed in order to be able to investigate the extent of C-mannosylation. It was shown that this process occurs almost exclusively where cell-cell communication is essential, especially in cytokine receptors of the immune system and adhesion GPCRs. The latter serve as "sensory antennae" for growing neurons that make their way through the brain.
"The topic is red-hot, especially for our understanding of the cell-cell communication of the immune system," explains Kaspar Locher, Professor of Structural Biology at ETH Zurich: "Signaling molecules such as cytokines direct the immune response during an infection. While these and their associated receptors have been intensively studied for decades, it has long been neglected that C-mannosylation determines whether a cytokine receptor reaches the cell surface to exert its function."
"With our insights into the structure of the enzymes involved, we now have a near-complete understanding of how C-mannosylation gets to these receptors," adds study first author Joël Bloch, former senior scientist in Locher's group.
Tailor-made molecular construction kit
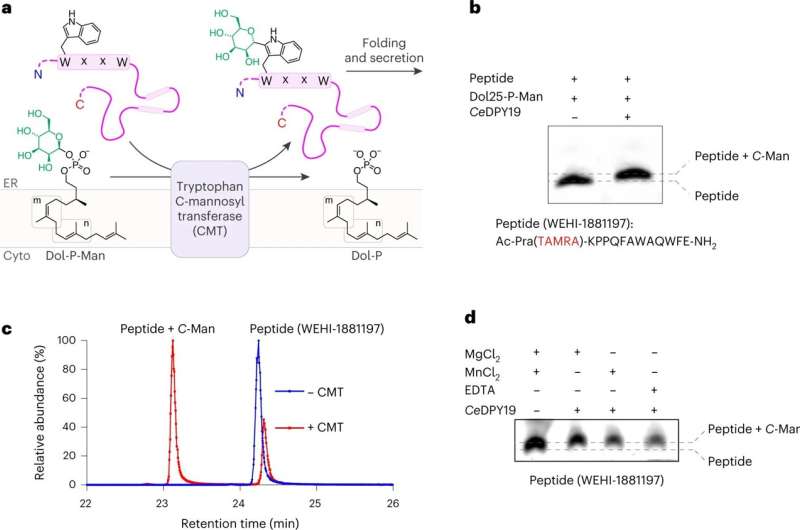
The ETH researchers succeeded in producing the CMT enzyme in its pure form. With the help of chemists from WEHI (AUS) and the University of Bern, they built customized molecules that mimic CMT-specific protein sequences and sugar substrates. This allowed them to test the enzyme for its specific properties in the test tube for the first time.
The researchers quickly realized that the enzyme chemistry of CMT must be novel and completely different from that of OST. "In such a case, we can only find out the mechanism of an enzyme using high-resolution structural elucidation. The problem, however, was that CMT could not be crystallized until now and had too little mass for cryo-EM, because this technique is particularly difficult to apply to proteins below 100 kDa," Locher explains.
Antibody enables high-resolution electron microscopy
A trick finally brought the breakthrough: In collaboration with researchers from the University of Chicago, the ETH scientists produced a synthetic antibody that binds specifically to the CMT. This antibody increased the mass of the enzyme so much that its structure could be elucidated with the help of cryo-EM. With the help of the cryo-EM structures, the group led by Kaspar Locher was finally able to decipher how the different CMT variants recognize different protein sequences.
Based on these insights, the researchers could now predict more precisely which proteins in humans carry the modification. From this, they hope to be able to capture the 'C-mannosyl proteome' in the near future.
By deciphering the peptide binding mechanism of CMTs, the researchers also hope to make progress in the production of CMT-specific inhibitors. Such molecules could contribute to advances in drug production, such as those to combat the malaria pathogen Plasmodium falciparum, which has its own CMT and needs it to attach to the host.
The sequence and organ specificity of the human CMT variant CMT2 could also be used, as it plays a key role in sperm development. The new findings could therefore be used to develop CMT2 inhibitors as contraceptives for men.
A novel enzyme mechanism
Another enigma for scientists was the enzymatic mechanism of CMT. This creates a unique carbon-carbon bond between protein and sugar. Using a custom-made CMT inhibitor molecule, the scientists were able to "capture" CMT in the middle of a glycosyl transfer reaction and elucidate a cryo-EM structure of it.
This allowed them to visualize the CMT reaction mechanism: a previously unknown form of electrophilic aromatic substitution enabled by precisely arranged side chains. Such insights could contribute to the development of designer enzymes that catalyze bonds between carbon atoms.
Evolutionarily conserved protective mechanism in glycosyltransferases
With a total of four different structures of the CMT, the scientists succeeded for the first time in visualizing a practically complete catalytic cycle of an enzyme of the GT-C superfamily.
In the process, they uncovered an astonishing mechanism: The sugar substrates of the CMT are complex to produce due to their lipid binding and are therefore particularly valuable. As it turned out, the CMT initially binds them in a non-reactive protected binding pocket. Only when the protein or peptide to be modified docks onto the CMT is the sugar substrate shifted by a peptide sensor and brought into a highly reactive state.
The scientists assume that this mechanism is evolutionarily conserved in GT-C enzymes and prevents valuable substrate molecules from being prematurely consumed. "Having uncovered the general architecture of GT-C enzymes three years ago, we now have a holistic understanding of their enzyme chemistry. It is another milestone in glycobiology," explains Locher.
More information: Joël S. Bloch et al, Structure, sequon recognition and mechanism of tryptophan C-mannosyltransferase, Nature Chemical Biology (2023). DOI: 10.1038/s41589-022-01219-9
Provided by ETH Zurich
Citation: A breakthrough in understanding the sugar biology of multicellular organisms (2023, January 16) retrieved 16 January 2023 from https://ift.tt/d7lwvha
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.
A breakthrough in understanding the sugar biology of multicellular organisms - Phys.org
Read More

No comments:
Post a Comment